CERT IND
ISO QUALITY SYSTEM
The Best Certification Decision and Re-certification

Our Story
To ensure the enhancement and continual improvement in the operational and quality efficiency through the value added, competitive, result oriented auditing practices to achieve international recognition for excellence of Management System Certification.

Our Story
To ensure the enhancement and continual improvement in the operational and quality efficiency through the value added, competitive, result oriented auditing practices to achieve international recognition for excellence of Management System Certification.
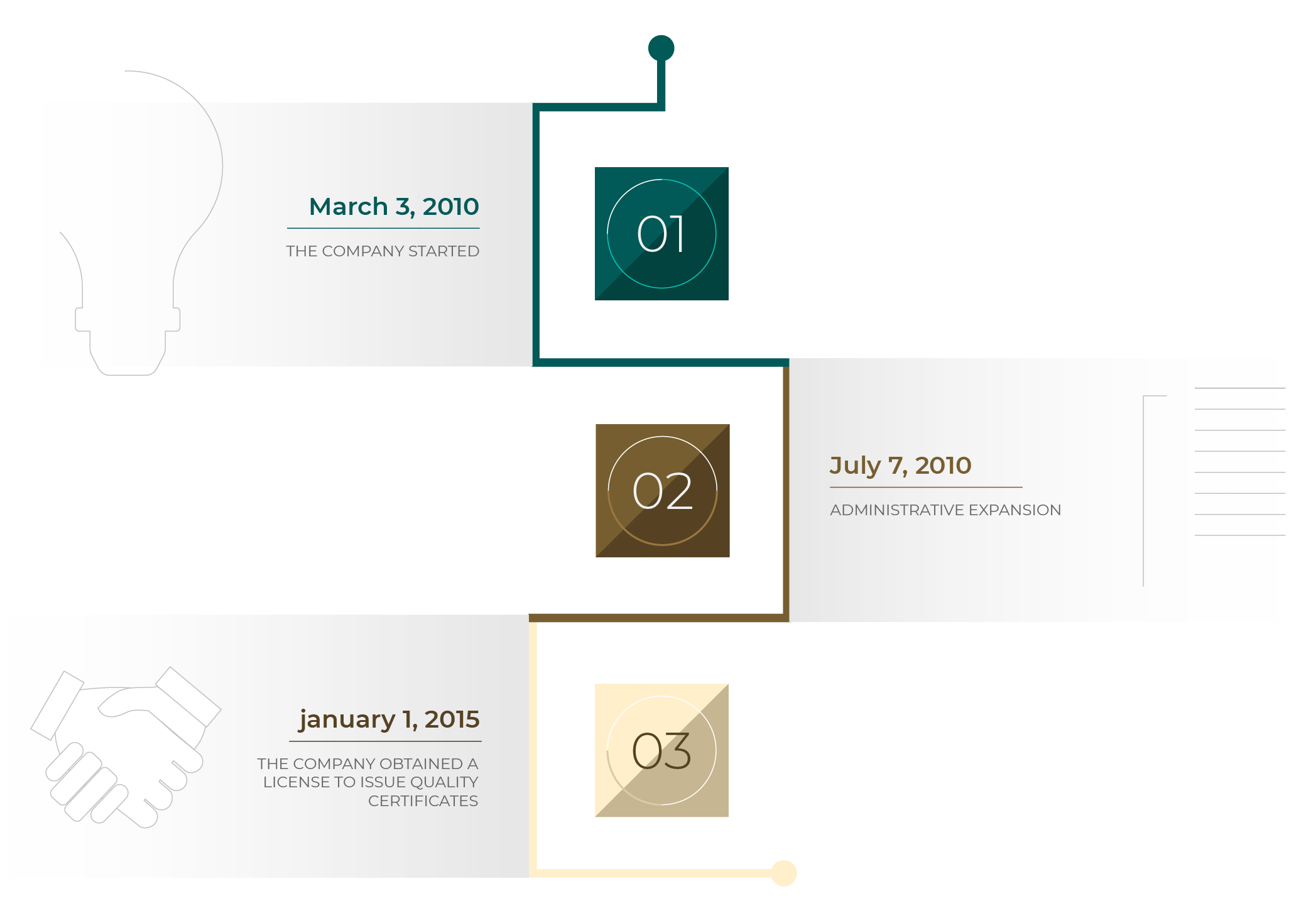
Time Line
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer malesuada efficitur consequat. Duis arcu diam, malesuada ac viverra sed, auctor ac urna. Nulla posuere mollis mi, vitae elementum nibh vulputate porttitor.
Our Clients





